Medical doctors often use the term biologics to refer to natural products that are harvested and used to augment a medical process. These products include tissues from a patient’s own body (often called autograft) and tissues from another patient’s body (often called allograft). These products can encompass a wide spectrum of tissues.
For the purposes of orthopedics, therapies can be classified into three main categories:
- Growth Factory Therapies
- Cell Therapies
- Tissue Therapies
Growth factor therapies involve the harvest and delivery of growth factors to a site, such as in the setting of using platelet-rich plasma to augment healing after a partial tear of a tendon. Cell therapies involve the harvest and delivery of cells to a site, such as in the setting of cartilage repair with peripheral blood stem cells. Tissue therapies involve utilizing tissue to repair or augment a repair, such as in the setting of a meniscal allograft transplant. Many factors have effects on function, the potential for success, and the FDA regulatory concerns related to these treatments. There is a spectrum of treatments available within biologics. Similar to golf clubs in a golf bag, there are different therapies available for different patient problems.
Food and Drug Administration Regulation
It is important to understand the regulatory affairs concerning biologics in order to understand the potential for how they can be used to help patients. This is especially important for stem cell therapies, as cells are living biologic products.

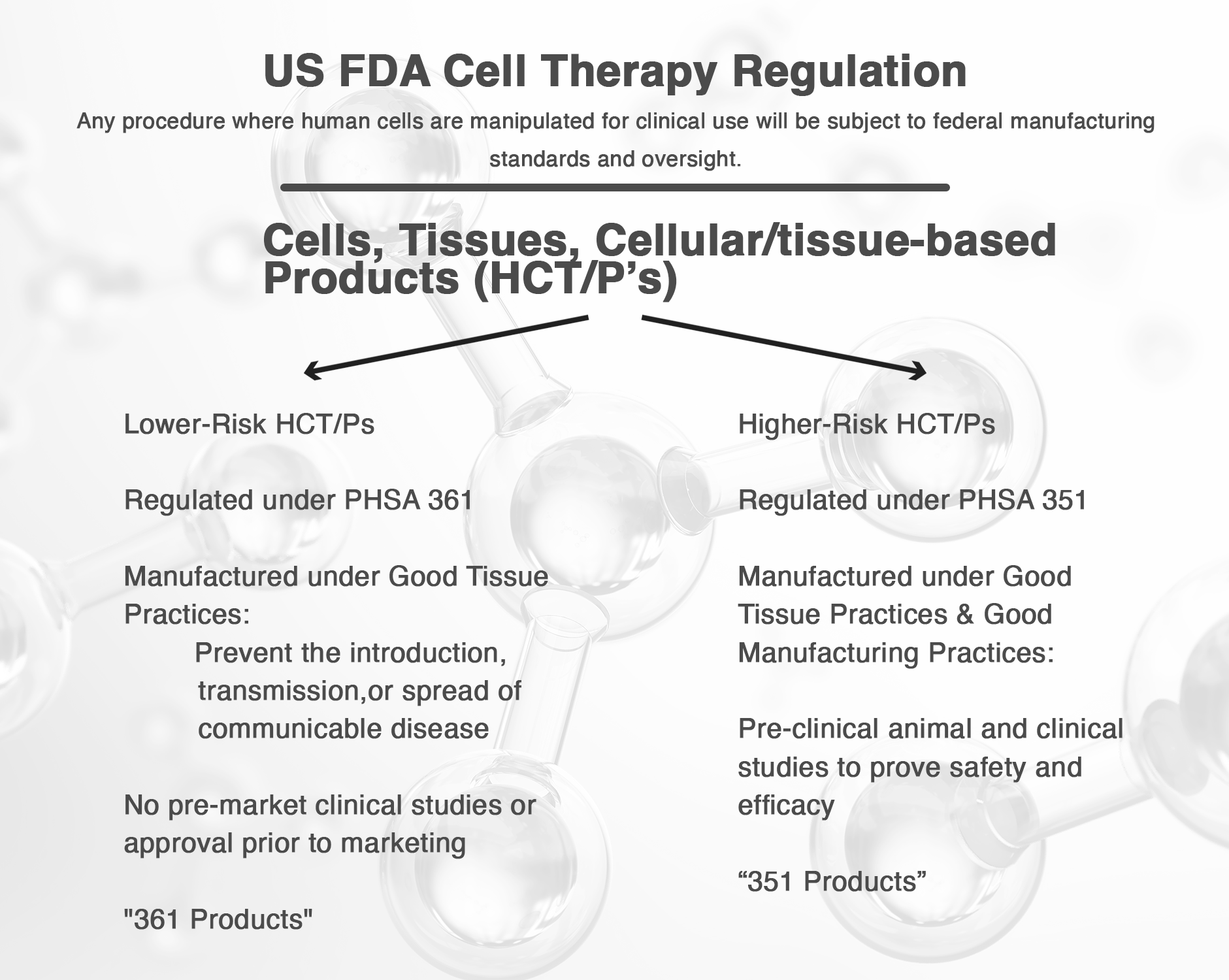
In 1997, the United States Food and Drug Administration (FDA) set forth in the Code of Federal Regulations Title 21 Part 1271, an approach to articles containing or consisting of all human cells, tissues, and cellular/tissue based products intended for implantation, transplantation, infusion, or transfer into a human recipient. These articles were abbreviated HCT/P’s, and the FDA employed a tiered approach to regulation of these articles, based on the FDA’s assessment of patient risk.
Low-risk biologics were set to be regulated by the Public Health Service Act 361 which required only that treatments be prepared involving techniques aimed to prevent the introduction, transmission, or spread of communicable diseases. These products do not require pre-market clinical studies or approval before they can be offered to patients.
Higher risk products are regulated under Public Health Service Act 351, whereby they must also undergo animal and human clinical studies in order to prove that they are safe and worthwhile to be used in patient care.
This hurdle prevents physicians from offering some stem cell treatments to patients such as cultured stem cell treatments, certain stem cell treatments from fat and blood, and stem cell treatments involving allograft stem cells.